USDA BioPreferred Program Starts 2018 Product Audit in May
The United States Department of Agriculture (USDA) will begin its BioPreferred® Program audit activities in May 2018. The USDA BioPreferred Program will email all participants to request that they complete an online Audit Declaration of Conformance. Participants are given 30 days to respond to the audit request. The Program expects the auditing process to be completed by October 2018.
The USDA BioPreferred Program recognizes about 15,000 products. To maintain the credibility of the Program, products are routinely audited to check that they are still eligible for the initiatives and the contact and product information of about 3,000 companies in the BioPreferred Catalog are kept updated.
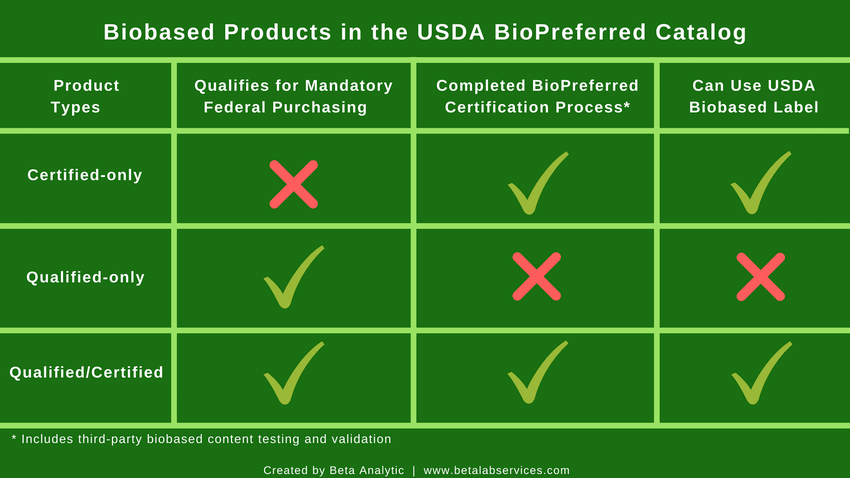
UPDATE: There are currently three groups of biobased products under the Program as of June 2022:
- Certified-only – Biobased products that do not fit into one of the 139 categories qualified for mandatory federal purchasing but have gone through the Program’s certification process, including third-party ASTM D6866 biobased content testing and validation.
- Qualified-only – Biobased products that fall within a mandatory federal purchasing category but have not gone through the USDA BioPreferred Program’s certification process nor third-party biobased content testing.
- Qualified/Certified – Products that belong in one of the 139 product categories qualified for mandatory federal purchasing AND have gone through the Program’s certification process, including third-party biobased content testing and validation.
Only the manufacturers of Certified-only and Qualified/Certified biobased products can use the USDA Certified Biobased Product Label on their packaging and marketing materials. The biobased content of a product appears on this label.
Ready to send your biobased samples?
Please read the lab’s instructions for USDA Certified Biobased Product Label Applicants
USDA BioPreferred Program 2018 Audit
 The USDA BioPreferred Program requests all participants to update their company and contact information, confirm if their product is still being manufactured, provide necessary updates, verify that the formulation and manufacturing process of their products have not changed since certification, and disclose any complaints received regarding their certified products’ biobased content claims.
The USDA BioPreferred Program requests all participants to update their company and contact information, confirm if their product is still being manufactured, provide necessary updates, verify that the formulation and manufacturing process of their products have not changed since certification, and disclose any complaints received regarding their certified products’ biobased content claims.
While the Program expects the auditing process to run until October, participants only have 30 days to respond to the audit request. The products of participants who fail to respond to the audit request will be removed from the Program’s database and online catalog.
The USDA BioPreferred Program will send a Notice of Removal from the database on June 15, 2018, to non-responding participants with qualified-only products.
Participants with certified-only and qualified/certified products who fail to respond would receive a Preliminary Notice of Violation on May 28, 2018. If they remain unresponsive to the Program’s communications, they will receive a Formal Notice of Violation on June 28, 2018; a Notice of Suspension on September 28, 2018; and final Notice of Revocation on October 28, 2018.
Source: USDA BioPreferred Program 2018 Product Audit Overview (last accessed April 25, 2018)
Beta Analytic is not affiliated with the USDA BioPreferred Program. However, the ISO 17025-accredited lab welcomes inquiries on how to submit samples for testing as part of the Program. Please contact the lab for turnaround time or price inquiries.
This entry was posted on Wednesday, April 25th, 2018 and is filed under Biobased Products, USDA Biopreferred Program .